We are proud to present you the first published results from CHAIR! The work of Antonio Pulcinella (ESR 11) and his colleagues has been accepted in Angewandte Chemie, under the title Rapid and Direct Photocatalytic C(sp3)−H Acylation and Arylation in Flow. The publication is Open Access and you can read it here.
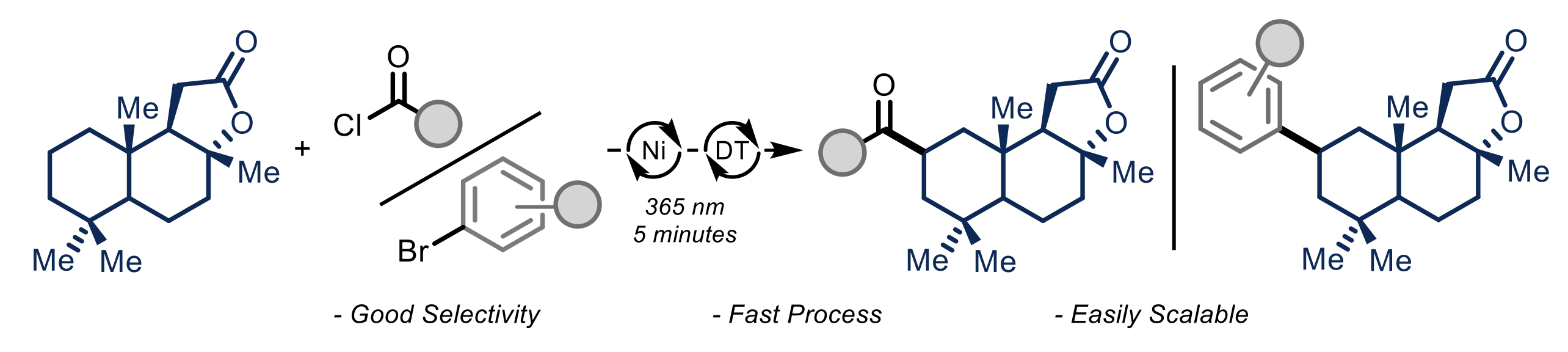
New methodologies capable of converting abundantly available hydrocarbon feedstocks into added-value products, thus effectively bypassing additional pre-functionalization steps, are highly appealing for the synthetic organic chemistry community. CHAIR ESR Antonio Pulcinella and his colleagues from University of Amsterdam recently developed a methodology capable of exploiting the synergic action of a hydrogen atom transfer catalyst, namely TBADT (decatungstate anion), and a nickel catalyst to achieve the selective functionalization of inactivated hydrocarbons in a flow reactor. In batch, these methods are generally plagued by long reaction times (12-48 h) and are not easily scalable, hence/therefore limiting a potential industrial application.
In this context, Antonio reported the direct acylation and arylation of strong aliphatic C−H bonds in a high power photochemical reactor. Thanks to the short optical path of the microflow reactor, providing a homogeneous irradiation, and an adequate light intensity, the desired transformations were achieved in only 5-15 minutes at ambient temperature. In addition, kinetic studies highlighted how the optimized conditions do not change the reaction mechanism but effectively speed up the overall transformation.