The Project
Research
Research Axis #1: Molecular Diversity via Direct Functionalization
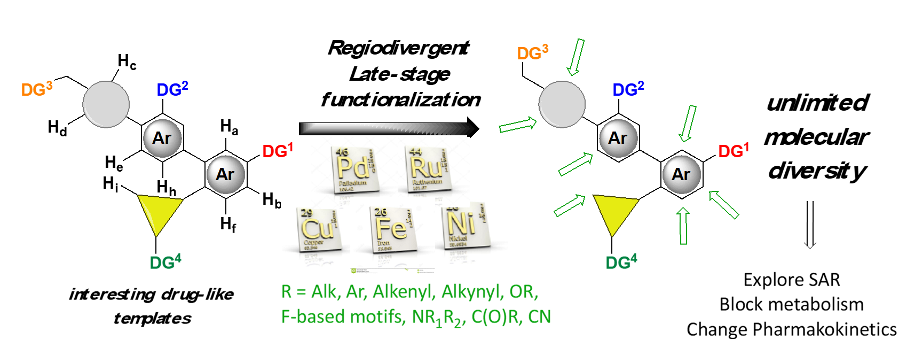
Objective: speed up the synthesis of drug candidates for structure-activity studies and devise new synthetic protocoles to rapidly convert a molecule into a large panel of biologically relevant derivatives, including regioisomeric and enantiopure compounds.
Research topics:
- late-stage diversification of advanced molecules
- remote functionalization using transient directing groups synthesis of chiral aliphatic motifs via asymmetric C-H activation
ESR: Dehang Yin
PI: Dr. J. Wencel-Delord
Host institution: CNRS / Unistra
Secondments: AstraZeneca, Janssen
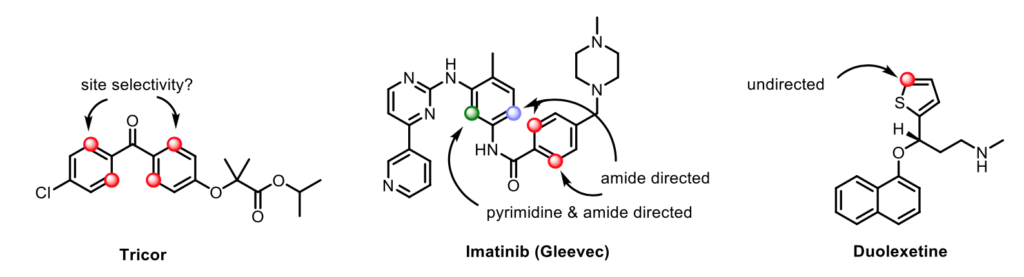
The aim of the project is to develop new chiral ligands promoting highly stereo-discriminant metalation of small alkane motifs of interest for the industrial partners. The attention will be focused on direct enantioselective functionalization of scaffolds bearing cycloalkane motifs. Once functionalization of simple substrates is achieved, the newly developed catalytic systems will be used, in collaboration with AstraZeneca and Syngenta, for late stage functionalization drug- and agrochemical-like molecules.
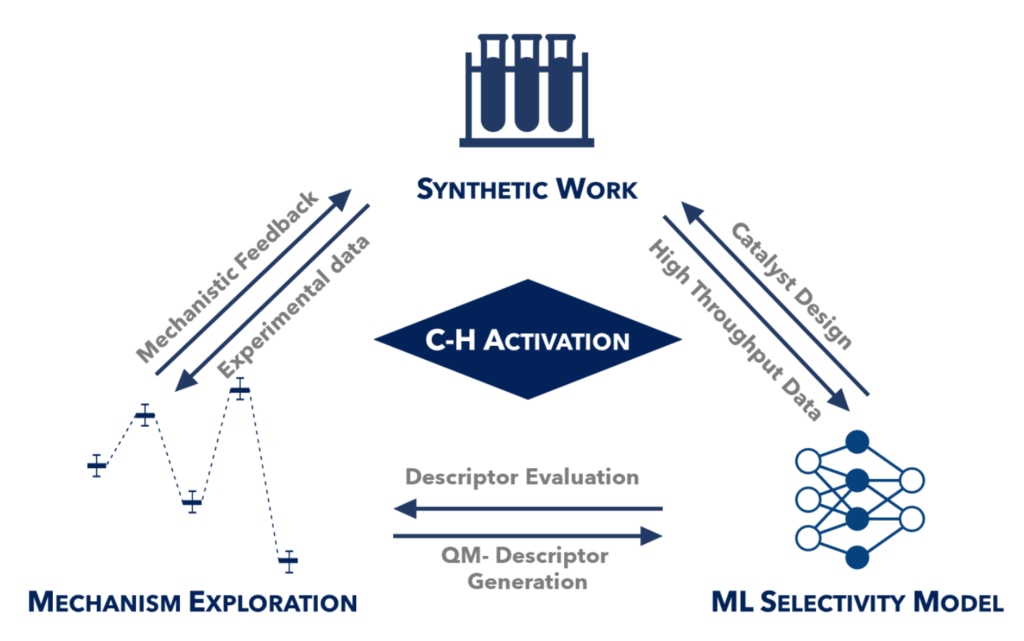
The project focuses on the fundamental modelling and mechanistic studies on transient templates for remote C-H activation, from both a synthetic and computational point of view. Tsuyoshi’s aim is to simulate chiral ligands prompt to induce chirality in direct C–H transformations, re-optimize ligands in terms of enantiomeric excess and yield following experimental results; identify key parameters controlling asymmetric C–H activation, and design a library of chiral ligands for asymmetric C–H activation for the generation of predictive models by ML to predict novel and highly efficient chiral ligands.
Secondments: UGOE, IST
This project aims to discover new synthetic transformations for chemo-selective C-H functionalization, using earth abundant catalysts, with a focus on commercial drugs or drug-like motifs.
Late stage functionalization will be achieved using a specific coordinating moiety inherently present in a scaffold, and employing known literature protocole: couplings (e.g. arylation, acetoxylation, halogenation, etc.) will be performed to access a large library of analogues. High throughput experimentation will be performed to identify suitable catalytic systems. The biological activity along with physical, chemical and DMPK properties of newly accessed derivatives will be assessed.
ESR: Janis Zakis
PI: Dr. T. Šmejkal
Host institution: Syngenta
Secondments: UGOE, UZH
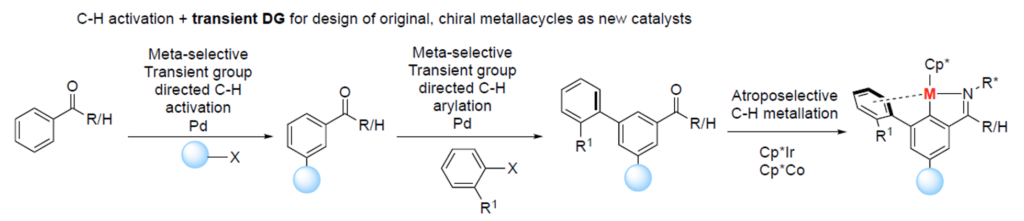
Novel catalytic methodologies will be developed using innovative (asymmetric) C-H activation strategies, including transient Directing Group (DG) approach. In close collaboration with Tsuyoshi Oyama (ESR 3), Janis works on the design of transient DGs (in-situ temporarily installed on a substrate to allow metalation at meta-position); the elucidation of key parameters governing regioselectivity of such transformations; the meta-selective functionalization, followed by recovery of a transient DG; the design of a library of several transient meta- and para-directing auxiliaries; the development of a protocol compatible with 3d metals (in collaboration with Takuya Michiyuki (ESR 9)); and the application of the developed catalytic systems for rapid exploration of the chemical space enabling biological screen and structure-activity relationships.